- General Dermatology
- Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
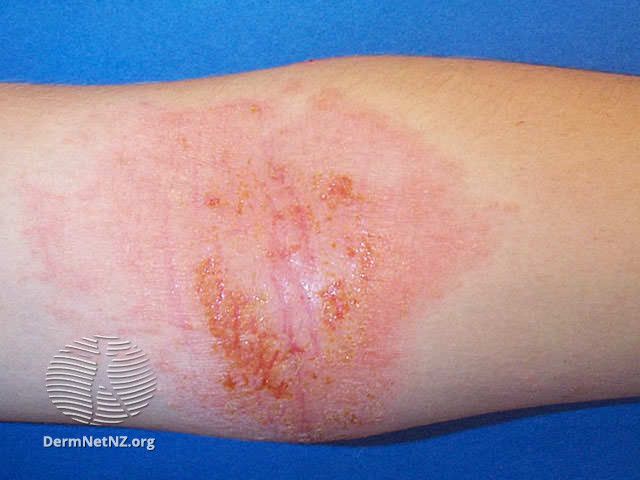
FDA Accepts Dermavant’s sNDA for Tapinarof Cream 1% for Adults and Children With AD
The FDA’s PDUFA date is expected in quarter 4 of 2024.
Dermavant Sciences recently announced that the US Food and Drug Administration (FDA) has accepted its supplemental new drug application (sNDA) for tapinarof (Vtama) cream 1% for the treatment of atopic dermatitis in adults and children aged 2 years and older. The FDA’s Prescription Drug User Fee Act (PDUFA) date is expected in the fourth quarter of 2024.1
The submission of the sNDA is based on data from the phase 3 ADORING 1 (NCT05014568) and ADORING 2 (NCT05032859) clinical trials, 2 identical, double-blind, randomized, vehicle-controlled, pivotal trials, as well as an open-label maximal usage pharmacokinetics trial in children aged 2 to 17 years, and the interim analysis results of ADORING 3, a phase 3, 48-week, open-label, extension trial.
"The landscape of atopic dermatitis (AD) management is evolving, offering hope for the millions affected, especially children. Currently, over 9.6 million kids in the US struggle with AD, experiencing relentless itching and scratching. However, finding safe and effective treatments, especially ones without steroids, remains a significant challenge," said John C. Browning, MD, FAAD, FAAP, a board-certified dermatologist at Texas Dermatology and Laser Specialists, adjunct associate professor at UT Health San Antonio, an assistant professor at Baylor College of Medicine, and clinical faculty at UIW School of Medicine, in a statement to Dermatology Times.
Browning added, "The collective findings of ADORING 1 and 2 studies highlight the potential of Vtama cream, 1% as a topical, non-steroidal treatment option for adults and children, as young as 2 years old, with atopic dermatitis, as well as for the physicians who treat them. Vtama cream has already brought about a shift in the treatment approach for plaque psoriasis, and I am eager to learn more about how it could benefit atopic dermatitis patients in the long run, and potentially simplify the treatment landscape by providing non-steroidal options that are both safe and effective long-term."
Notably, tapinarof cream is a novel, aryl hydrocarbon receptor agonist and a steroid-free topical. For atopic dermatitis, tapinarof cream will be useful for acute treatment and long-term management.
“The FDA acceptance of our sNDA is an important milestone in our efforts to bring Vtama cream, as a potentially safe and well-tolerated non-steroidal treatment. option, to adults and children as young as 2 years old who suffer from atopic dermatitis. Our commitment to patients is unwavering, and we remain highly focused on preparing for the commercial launch of Vtama cream, subject to FDA approval, for its second indication of atopic dermatitis,” said Todd Zavodnick, Chief Executive Officer of Dermavant, in the news release.
Tapinarof cream was approved by the FDA in May 2022 for the treatment of plaque psoriasis, making the topical the first non-steroidal topical novel chemical entity launched for psoriasis in the US in more than 25 years. The strength and formulation of tapinarof cream approved for the treatment of plaque psoriasis are the same as what is being studied in the ADORING phase 3 development program.
“Nonsteroidal topicals, like Vtama cream, have really challenged the treatment paradigm and I have seen a shift in my personal dermatology practice where I reach for Vtama over topical corticosteroids... As someone who sees the physical and emotional impact that chronic skin conditions can have on patients daily, I’m excited about the innovations we’re witnessing within the topical treatment landscape,” said Mona Shahriari, MD, assistant clinical professor of dermatology at the Yale School of Medicine, the associate director of clinical trials at Central Connecticut Dermatology Research, and a Dermatology Times Editorial Advisory Board member, in a recent interview.
In the interim analysis of the ADORING 3 study2, Dermavant reported that efficacy continued to improve beyond the 8-week mark. In total, 51.2% of patients (n=373), achieved complete disease clearance as determined by a Validated Investigator Global Assessment for Atopic Dermatitis (vIGA-AD) score of 0.2
In total, 73% of patients (n=519) achieved a vIGA-AD score of clear or almost clear with at least 2 grades of improvement. Additionally, 92.3% of patients (n=656) achieved at least a 1-point improvement in vIGA-AD scoring. Eczema Area and Severity Index (EASI) scores also improved. Approximately 80.7% (n=574) of patients achieved EASI-75.
Regarding itch, an average reduction was reported as early as 24 hours after tapinarof cream application, with 77.9% (n=218) of patients who had presented with a Peak Pruritus Numeric Rating Scale (PP-NRS) score of equal to or greater than 4 achieving a minimum 4-point PP-NRS score reduction.
Tapinarof cream was also well-tolerated among patients with no new safety signals reported. Adverse events were predominantly mild to moderate in nature, with no serious events related to treatment reported. The discontinuation rate attributed to adverse events was low at 2.6%.
References
- Dermavant announces FDA acceptance of supplemental new drug application (sNDA) for VTAMA (tapinarof) cream, 1% for the treatment of atopic dermatitis in adults and children 2 years of age and older. News release. Dermavant Sciences. April 29, 2024. Accessed April 29, 2024. https://dermavant.com/dermavant-announces-fda-acceptance-of-supplemental-new-drug-application-snda-for-vtama-tapinarof-cream-1-for-the-treatment-of-atopic-dermatitis-in-adults-and-children-2-years-of-age-and-old/
- Andrus E. Dermavant shares positive safety and efficacy data from analysis of ADORING program for atopic dermatitis. Dermatology Times. January 11, 2024. Accessed April 29, 2024. https://www.dermatologytimes.com/view/dermavant-shares-positive-safety-and-efficacy-data-from-analysis-of-adoring-program-for-atopic-dermatitis