- General Dermatology
- Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
Abrocitinib Approved for Adolescents With Atopic Dermatitis
The expanded approval is based on data from a recent phase 3 clinical trial.
AmpYang Images/AdobeStock
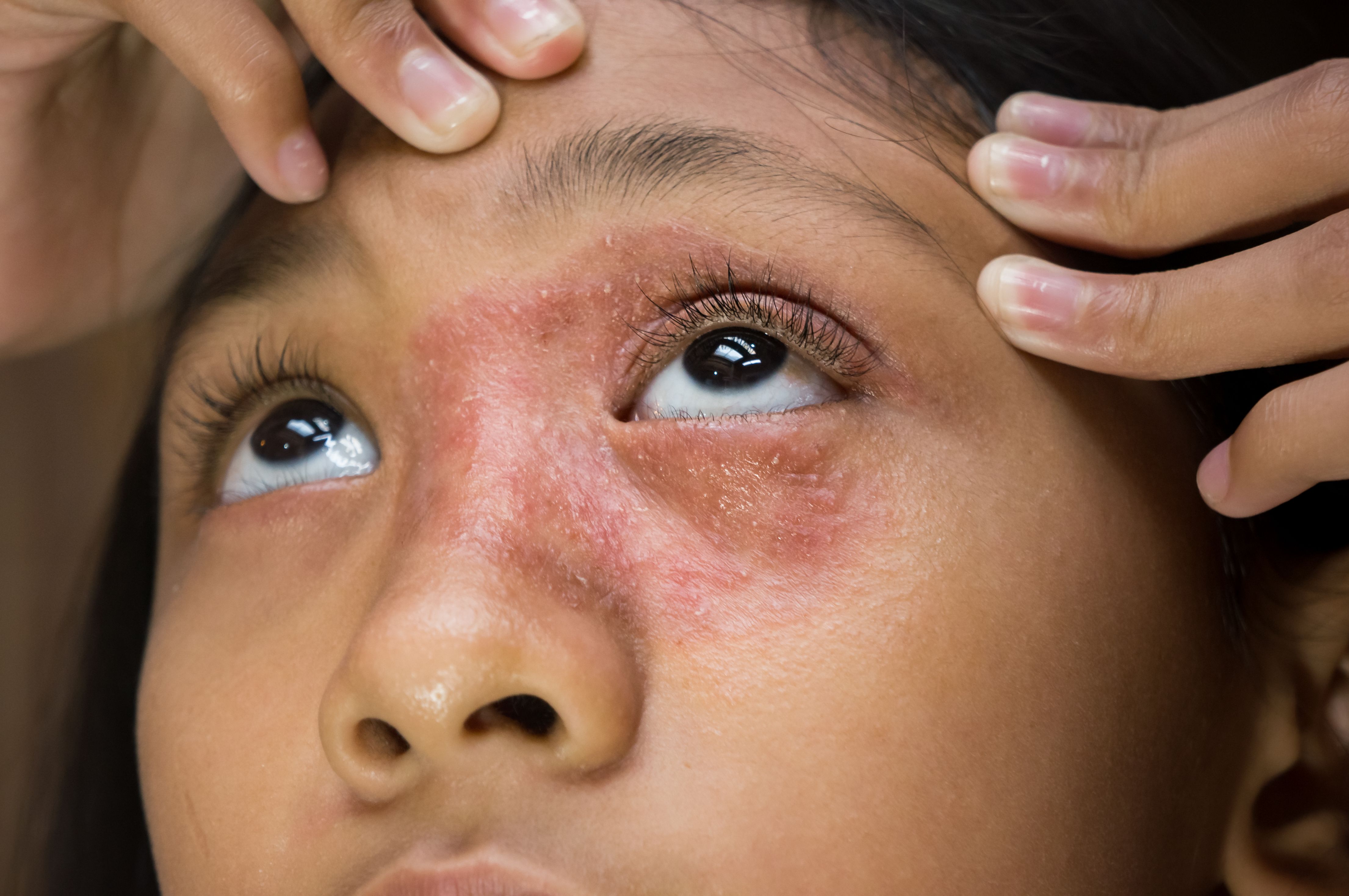
US Food and Drug Administration (FDA) has approved a supplemental new drug application to expand the use of abrocitinib (Cibinqo, Pfizer) for adolescents with moderate to severe atopic dermatitis (AD) when other systemic drug products are not adequate or advisable.1
Abrocitinib is an oral Janus kinase (JAK) 1 inhibitor, which is hypothesized to modulate multiple cytokines involved in the pathophysiology of AD, including interleukins (IL)-4, IL-13
IL-31, IL-22, and thymic stromal lymphopoietin. It was previously approved only for the treatment of AD in patients 18 years and older.
The approval comes after data from the JADE TEEN phase 3 clinical trial showed positive results. The trial included 285 adolescents aged 12 to 17 with moderate to severe AD. Patients received 100 mg or 200 mg of abrocitinib or placebo, randomly, once a day for 12 weeks while also on topical therapy. The results showed
- 39% of patients on the 100 mg dose, 46% on the 200 mg dose and 24% on the placebo achieved an Investigator Global Assessment response rate of 0 to 1.
- 64% of those on 100 mg, 71% of those on 200 mg, and 41% of those on placebo achieved a 75% improvement in their Eczema Area and Sensitivity Index scores.
- 13% of those on the 100 mg dose, 25% of those on 200 mg, and 8% of those on placebo achieved at least a -4 point decrease from baseline in their Peak Pruritis Numerical Rating Scale scores.
The most common adverse events reported in ≥1% of patients administered abrocitinib for up to 16 weeks included nasopharyngitis (12.4% with abrocitinib), nausea (6%), and headaches (6%).
Abrocitinib is not recommended for use with other JAK inhibitors, biologic immunomodulators, or other immunosuppressants.
Reference
1. FDA Approves Pfizer's Supplemental New Drug Application for CIBINQO® (abrocitinib). Pfizer. News release. https://www.pfizer.com/news/press-release/press-release-detail/fda-approves-pfizers-supplemental-new-drug-application. Accessed February 14, 2023.
